OpenAI Multi-AI Agent Research Framework
If you want to gain a better understanding of how a multi-AI Agent system look in practical terms, this will help you.
I took an OpenAI notebook at got it working in a Colab environment….
Key Considerations for Building Agentic Applications
While prototyping AI agents using OpenAI’s notebooks, I stumbled onto their research API and model — it opened up some intriguing thoughts for me in terms of AI Agents…
Complexity has to reside somewhere in your system.
You could offload it to the model provider, letting them handle the heavy lifting for a fee, but that often means sacrificing fine-grained control over how things work.
For enterprises, though, it makes sense to pull that complexity into your own stack and manage it internally.
That’s where real efficiencies kick in — customising to your needs without relying on black-box solutions.
The notebook I’ve shared below demonstrates an agentic application that coordinates a team of AI Agents, each handling a distinct role.
Every agent is built around a tailored language model, chosen to align with the task’s demands in terms of cost and sophistication.
This way, you’re not overkilling simple jobs with heavyweight models or skimping on complex ones.
In this setup, I lean on research-oriented AI gents powered by a dedicated research API and model, rather than general-purpose ones.
A couple of trade-offs to note…
First, responses from this API and model take noticeably longer and likely come at a premium (though I haven’t crunched the numbers on costs yet).
Second, the results are richly detailed and comprehensive, which explains the higher latency — it’s doing deeper reasoning under the hood.
This raises a core dilemma in designing agentic apps: Should the entire research process be delegated to the model’s built-in capabilities, or embedded as custom code in the agent itself?
It’s all about striking the right balance — how much do you tap into the model’s off-the-shelf features versus rolling your own to fit your exact workflow?
Working Notebook
I had to make some changes to the notebook shared by OpenAI to get it to work…
Run the initial installs…
%pip install --upgrade "openai>=1.88" "openai-agents>=0.0.19"I set my OpenAI API key in the secrets functionality of Colab…
The system disables Data Retention through the os.environ setting below, enabling enterprises to operate in a Zero Data Retention environment with Deep Research.
If Data Retention is not an active constraint for users, they should consider keeping it enabled to benefit from automated traceability for their AI Agent workflows and deep integration with other platform tools, such as evaluations and fine-tuning.
import os
from agents import Agent, Runner, WebSearchTool, RunConfig, set_default_openai_client, HostedMCPTool
from typing import List, Dict, Optional
from pydantic import BaseModel
from openai import AsyncOpenAI
from google.colab import userdata
# Use env var for API key and set a long timeout
client = AsyncOpenAI(api_key=userdata.get('OPENAI_API_KEY'), timeout=600.0)
set_default_openai_client(client)
os.environ["OPENAI_AGENTS_DISABLE_TRACING"] = "1" # Disable tracing for Zero Data Retention (ZDR) OrganizationsBasic Deep Research Agent
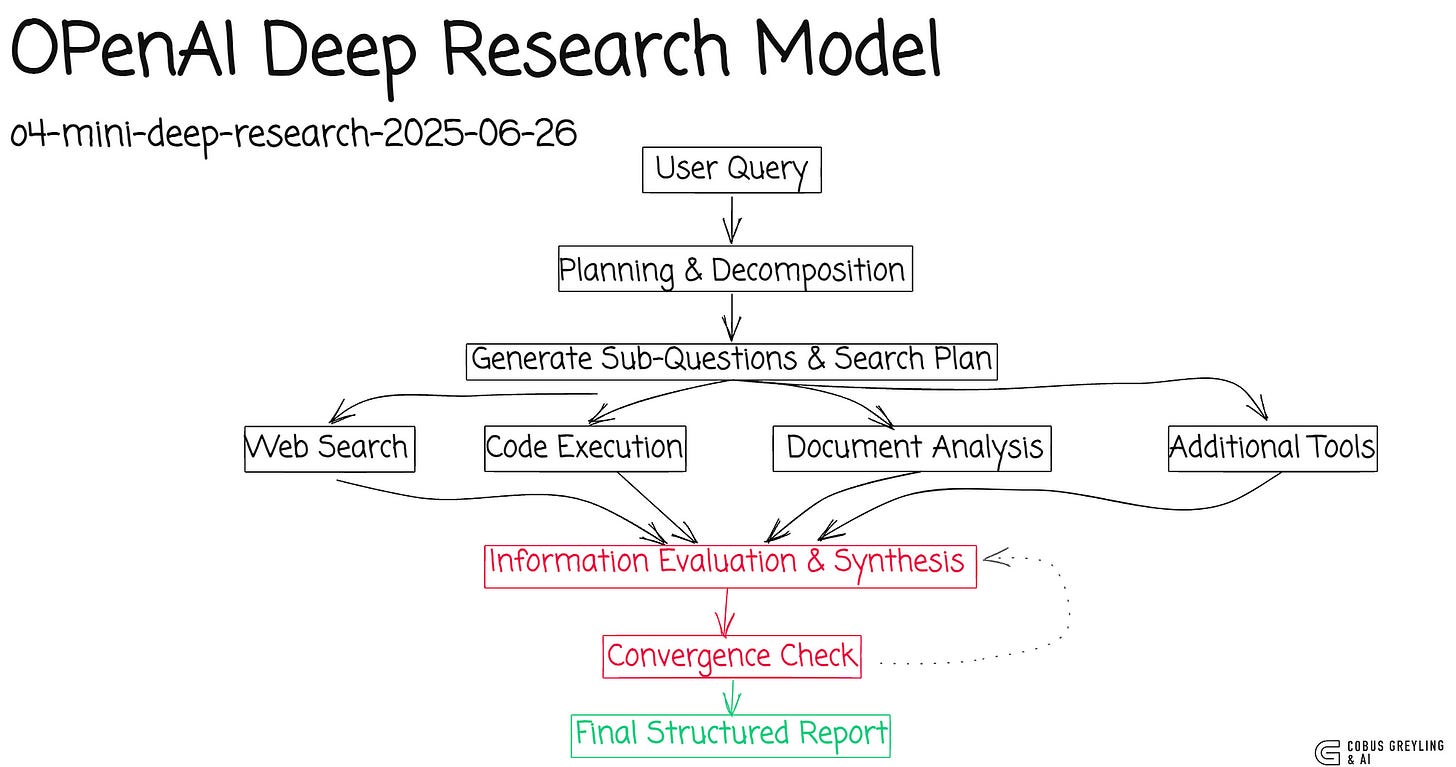
The Basic Research Agent performs Deep Research using the o4-mini-deep-research-alpha model.
It has native WebSearch access to the public internet and streams its findings directly back into the notebook.
In this case, the o4-mini-deep-research-alpha model is used because it is faster than the full o3 deep research model, with acceptable intelligence.
# Define the research agent
research_agent = Agent(
name="Research Agent",
model="o4-mini-deep-research-2025-06-26",
tools=[WebSearchTool()],
instructions="You perform deep empirical research based on the user's question."
)
# Async function to run the research and print streaming progress
async def basic_research(query):
print(f"Researching: {query}")
result_stream = Runner.run_streamed(
research_agent,
query
)
async for ev in result_stream.stream_events():
if ev.type == "agent_updated_stream_event":
print(f"\n--- switched to agent: {ev.new_agent.name} ---")
print(f"\n--- RESEARCHING ---")
elif (
ev.type == "raw_response_event"
and hasattr(ev.data, "item")
and hasattr(ev.data.item, "action")
and ev.data.item.action is not None # Add this check
):
action = ev.data.item.action
if action.type == "search":
print(f"[Web search] query={action.query!r}")
# streaming is complete → final_output is now populated
return result_stream.final_output
# Run the research and print the result
result = await basic_research("Research the economic impact of semaglutide on global healthcare systems.")
print(result)The output…
Researching: Research the economic impact of semaglutide on global healthcare systems.
--- switched to agent: Research Agent ---
--- RESEARCHING ---
[Web search] query='economic impact semaglutide global healthcare'
[Web search] query='semaglutide economic impact healthcare cost obesity diabetes'
[Web search] query='semaglutide healthcare cost global budgets impact'
[Web search] query='semaglutide global health economic impact system analysis'
[Web search] query='budget semaglutide UK NHS obesity cost'
[Web search] query='semaglutide NHS budget shortage injunction shortage cost obesity Denmark'
[Web search] query='cost of obesity global economy healthcare semaglutide'
[Web search] query='semaglutide WHO global cost access obesity inclusion'
[Web search] query='"Exclusive-WHO to back use of weight-loss drugs for adults globally"'
[Web search] query='semaglutide global healthcare budget shortages obesity insurance'
[Web search] query='"In only nine was obesity pharmacotherapy made available"'
[Web search] query='global semaglutide market size 2024 revenue'
# Overview of Semaglutide and Health Economics
Semaglutide (brand names Ozempic/Wegovy/Rybelsus) is a GLP-1 receptor agonist approved for type-2 diabetes and obesity management. It potently lowers blood sugar and body weight, reducing complications and improving outcomes. These clinical benefits offer the potential to decrease long-term healthcare use (e.g. fewer heart attacks or kidney disease), but semaglutide’s high price raises concerns about short-term costs. Global spending on diabetes care alone is enormous – roughly **US$966 billion in 2021** for adults worldwide ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC12094200/#:~:text=The%20economic%20burden%20of%20type,value%2C%20preventive%20health)) – so any effective intervention could offset parts of that cost. Nonetheless, semaglutide therapy currently costs on the order of **>$1,000 USD per month** ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=The%20World%20Health%20Organization%20,and%20may%20require%20lifelong%20usage)), which can strain health budgets if used broadly. Health economists therefore emphasize a careful balance: semaglutide may yield long-term savings by preventing obesity-related illness, but its **up-front expense** means healthcare systems must target use rigorously.
# Cost-Effectiveness Analyses
Numerous studies have evaluated semaglutide’s cost-effectiveness compared to other treatments. In type-2 diabetes, a 2025 systematic review/meta-analysis of 119 comparisons across Europe, North America, and China found semaglutide to be *dominant or cost-effective* in about **73.9% of trials** ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC12094200/#:~:text=DATA%20SYNTHESIS)). However, results often varied by study sponsorship and assumptions: industry-sponsored analyses uniformly showed semaglutide as cost-effective, whereas non-industry studies found it cost-effective only about half the time ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC12094200/#:~:text=DATA%20SYNTHESIS)). In general, semaglutide’s superior glucose and weight control translates into more *quality-adjusted life-years* (QALYs) gained than older glucose-lowering drugs, and in many models the extra health benefit justifies its cost.
For obesity management (in patients without diabetes), the picture is more mixed. One recent systematic review of cost-effectiveness in obese adults found that **semaglutide delivers more QALYs but at much higher cost** than alternative therapies ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/39254692/#:~:text=Conclusion%3A%20The%20current%20systematic%20review,sleeve%20gastroplasty%2C%20and%20gastric%20bypass)). The incremental cost-effectiveness ratio (ICER) often exceeds typical willingness-to-pay thresholds, meaning its price is the main barrier. For example, one analysis projected **lifetime per-person costs** of about **$370,000 USD** for semaglutide (BMI 33), versus ~$125,000–$140,000 for bariatric or other interventions ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/39254692/#:~:text=class%20I%20obesity%20,126%2C732%3B%20%24139%2C971%3B%20and%20%24370%2C776%2C%20respectively)). In summary, semaglutide can be cost-effective *only if* its price is substantially lowered or its use is limited to patients most likely to benefit.
Even in high-risk subgroups, semaglutide may not meet standard cost-effectiveness cutoffs at current prices. A Canadian modeling study of obese patients with cardiovascular disease (but without diabetes) found semaglutide’s ICER ≈ **C$72,962 per QALY**, exceeding the common threshold (~C$50,000) ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=is%20considered%20acceptable%20healthcare%20value,effectiveness%20at%20a%20CAN%2450%2C000%2FQALY%20threshold)). With this pricing it had just a 14% chance of being “worth it.” However, if its price were cut by **50%** the ICER fell to ~C$37,190/QALY (with an ~80% chance of being cost-effective) ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=is%20considered%20acceptable%20healthcare%20value,effectiveness%20at%20a%20CAN%2450%2C000%2FQALY%20threshold)). In short, without discounts, semaglutide often fails conventional cost-effectiveness tests in obesity. The pattern is consistent: at today’s list price it yields significant health gains, but the extra cost is high. If price drops into range (e.g. via generics or rebates), many studies predict it would then represent good value.
> **Key cost-effectiveness findings:**
> - *Type 2 diabetes:* Semaglutide was found dominant or cost-effective vs. other drugs in ~74% of models ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC12094200/#:~:text=DATA%20SYNTHESIS)).
> - *Obesity:* Compared to diet/exercise or older drugs (e.g. liraglutide), semaglutide adds QALYs but at ~$30k–40k extra cost per QALY ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/39254692/#:~:text=Conclusion%3A%20The%20current%20systematic%20review,sleeve%20gastroplasty%2C%20and%20gastric%20bypass)) ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=is%20considered%20acceptable%20healthcare%20value,effectiveness%20at%20a%20CAN%2450%2C000%2FQALY%20threshold)).
> - *Budget thresholds:* Many models fail to meet typical $50k/QALY benchmarks unless prices fall by roughly half ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=For%20overweight%20or%20obese%20individuals,An%20article%20in)) ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=is%20considered%20acceptable%20healthcare%20value,effectiveness%20at%20a%20CAN%2450%2C000%2FQALY%20threshold)).
# Health System Budget Impacts
Semaglutide’s adoption has significant budget implications for public and private payers. Even if it prevents costly complications later, broad subsidization entails **large upfront spending**. For example, in the UK the National Health Service (NHS) spent about **£107 million (~$135M USD)** on semaglutide injections in one year (making it the 11th largest drug cost) ([www.diabetes.co.uk](https://www.diabetes.co.uk/news/2023/jun/obesity-epidemic-drives-nhs-prescription-cost-past-10-billion.html#:~:text=Otherwise%20known%20as%20Ozempic%20and,th%7D%20most%20expensive%20prescription%20drug)). The UK government is now piloting broader use for obesity, but NICE restricts it to very obese patients: NHS guidance limits Wegovy (semaglutide) to those with BMI≥35 (or ≥30 with comorbidity) and only for up to 2 years ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)). This reflects the trade-off: NICE judged semaglutide cost-effective only under those strict conditions, given its high price.
In modeled scenarios, expanding access can produce substantial downstream savings. U.S. analysts using microsimulation have estimated that broad Medicare coverage of GLP-1 drugs could **save hundreds of billions** in future costs. For instance, one model projected that covering semaglutide for all eligible patients in Medicare would offset roughly **$245 billion** in Part A/B spending over 10 years (and $1.4 trillion over 30 years) by preventing comorbidities ([www.ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK609400/#:~:text=The%20scenario%20in%20which%20both,would%20lead%20to%20a%209)). Extending coverage to Medicaid and uninsured yields even larger “social benefits” – on the order of **$4 trillion in 10 years** ([www.ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK609400/#:~:text=disease%29,within%2020%20years%2C%20and%20over)). In these analyses, most savings came from avoided hospitalizations and less advanced disease. Thus, while annual drug spending would rise, the net effect could be a budgetary *break-even or gain* over decades.
Other countries face similar calculations. A 2024 budget-impact study in Saudi Arabia found that adding oral semaglutide to the diabetes program would increase immediate drug budgets, but lead to better patient outcomes ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/37933169/#:~:text=Budget%20impact%20of%20introducing%20oral,with%20diabetes%20an%20essential%20part)) ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/39254692/#:~:text=The%20cost,patients%20with%20obesity%20or%20overweight)). In public health terms, treating obesity and diabetes aggressively may reduce costs of related conditions (heart disease, kidney failure, etc.). One report by ING Bank estimated that UK obesity costs ~£100 billion per year (with £19bn on healthcare alone) ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=The%20ING%20healthcare%20analyst%20Diederik,and%20%E2%82%AC2%2C500%20in%20the%20US)). By comparison, a year’s supply of Ozempic costs only £830 in the UK ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=A%20year%E2%80%99s%20supply%20of%20Ozempic%2C,Netherlands%20and%20%E2%82%AC14%2C500%20in%20the)) – meaning even widespread semaglutide treatment could be cheaper than doing nothing. The ING analyst concluded that long-term savings from obesity drugs could vastly outweigh drug costs, if their weight-loss effects persist ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=%E2%80%9CIf%20the%20drugs%20are%20effective,save%20society%20money%2C%E2%80%9D%20he%20said)).
However, this optimistic view is tempered by realities of current budgets. In the UK, one productivity analysis found that each semaglutide-treated person gained ~5 extra workdays per year, translating to ~£1,127 in productivity value per person ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=A%20new%20study%2C%20presented%20at,well%20as%20reducing%20their%20consumption)). Across the eligible population this implied ~£4.5 billion/year in economic gains (plus smaller health savings) ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=Giving%20weight%20loss%20jabs%20to,5bn%2C%20according%20to%20research)) ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=A%20new%20study%2C%20presented%20at,well%20as%20reducing%20their%20consumption)). But experts cautioned these gains do not immediately pay for the drugs: the roughly **£2,500 per patient per year** cost (for Wegovy) means total drug spend would *exceed* these societal savings ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=society%20from%20weight%20loss%20medication,total%20health%20and%20societal%20gains)). In practical terms: treating every eligible person now is unaffordable, so most systems focus on **high-risk groups** (highest BMI or comorbidities) where cost-offsets are greatest ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=society%20from%20weight%20loss%20medication,total%20health%20and%20societal%20gains)) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=In%20terms%20of%20AOMs%E2%80%99%20inclusion,economic%20ones%2C%20to%20evaluate%20true)).
> **Budget impact examples:**
> - *United Kingdom:* NHS spent ~£107M on semaglutide in 2022/23 ([www.diabetes.co.uk](https://www.diabetes.co.uk/news/2023/jun/obesity-epidemic-drives-nhs-prescription-cost-past-10-billion.html#:~:text=Otherwise%20known%20as%20Ozempic%20and,th%7D%20most%20expensive%20prescription%20drug)) and restricts its use to BMI≥35 patients ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)). A study found £4.5 bn in potential productivity gains if all eligible were treated ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=Giving%20weight%20loss%20jabs%20to,5bn%2C%20according%20to%20research)), but drug costs far exceed those gains ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=society%20from%20weight%20loss%20medication,total%20health%20and%20societal%20gains)).
> - *United States:* Simulations indicate Medicare coverage of GLP-1 drugs could recover ~$245–704 bn in healthcare spending over 10–30 years ([www.ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK609400/#:~:text=The%20scenario%20in%20which%20both,would%20lead%20to%20a%209)). Early expanded coverage (Medicare + private) yields even larger offsets (>$1.4 tn in 30 years) ([www.ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK609400/#:~:text=The%20scenario%20in%20which%20both,would%20lead%20to%20a%209)).
> - *Saudi Arabia:* Introducing semaglutide into public formularies raises immediate costs but is projected to improve outcomes (analyses ongoing) ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/37933169/#:~:text=Budget%20impact%20of%20introducing%20oral,with%20diabetes%20an%20essential%20part)) ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/39254692/#:~:text=The%20cost,patients%20with%20obesity%20or%20overweight)).
# Global Market and Access Trends
Semaglutide’s economic impact unfolds in a **global market** that is rapidly expanding. The global weight-loss drug market (dominated by GLP-1 therapies like Wegovy/Ozempic) is estimated at **US$80–140 billion** ([www.ft.com](https://www.ft.com/content/3f03b203-72a8-4921-9ecc-0c9ea9ec0f62#:~:text=help%20treat%20obesity%2C%20which%20in,Hikma%E2%80%99s)). Soaring demand has already forced insurers and governments to ration access at current prices ([www.ft.com](https://www.ft.com/content/3f03b203-72a8-4921-9ecc-0c9ea9ec0f62#:~:text=help%20treat%20obesity%2C%20which%20in,Hikma%E2%80%99s)). For example, the FT reports that some European health systems and private payers are limiting semaglutide use due to cost ([www.ft.com](https://www.ft.com/content/3f03b203-72a8-4921-9ecc-0c9ea9ec0f62#:~:text=help%20treat%20obesity%2C%20which%20in,Hikma%E2%80%99s)). By contrast, manufacturers see blockbuster sales: Novo Nordisk’s Wegovy was prescribed to tens of thousands in H2 2024 ([www.ft.com](https://www.ft.com/content/dcc1e573-0b5d-4d3a-a581-abd93388ff76#:~:text=2024,The%20company%20is%20now%20prescribing)), and China and India have recently approved or are expected to approve it ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/novo-nordisk-says-semaglutide-approved-long-term-weight-management-china-2024-06-25/#:~:text=2024,key%20ingredient%2C%20is%20set%20to)) ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/demand-obesity-drugs-shoots-up-india-lilly-novo-jostle-market-share-2025-07-07/#:~:text=2025,doubling%20its%20sales%20in%20June)), tapping massive populations.
Patent expiries and generic competition are poised to reshape economics soon. Semaglutide’s composition will lose marketing exclusivity in major markets in **2026–2027** ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=As%20in%20HICs%2C%20out,reduce%20obesity%20and%20downstream%20comorbidities)) ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)). Generic manufacturers (e.g. Hikma) are developing copycat versions that typically cost **80–85% less than branded drugs ([www.ft.com](https://www.ft.com/content/3f03b203-72a8-4921-9ecc-0c9ea9ec0f62#:~:text=Hikma%2C%20a%20major%20drugmaker%2C%20is,Hikma%E2%80%99s)). When generics arrive, the price of semaglutide-like therapies could drop dramatically. The WHO and IQVIA anticipate that falling prices will be crucial for wider adoption, especially in lower-income countries ([globalsouthworld.com](https://globalsouthworld.com/article/exclusive-who-set-to-back-use-of-weight-loss-drugs-for-adults-globally-raises-cost-issue#:~:text=But%20it%20also%20notes%20that,in%20some%20markets%20next%20year)) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=For%20LMICs%2C%20people%20with%20obesity,or%20other%20forms%20of%20socio)). For instance, the WHO notes that semaglutide’s patent expiry will allow *cheaper generics* next year ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)). Lower-cost competition could enable many health systems to expand coverage beyond current high-risk limits.
Even without generics, some risk-sharing deals have emerged. For instance, the UK NHS negotiated outcomes-based agreements with manufacturers (like the one for tirzepatide) to cap costs for certain patient groups ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=In%20terms%20of%20AOMs%E2%80%99%20inclusion,economic%20ones%2C%20to%20evaluate%20true)). In the U.S., some insurers and Medicare pilots are beginning to cover GLP-1 drugs for obesity (e.g. Medicare covers older adults with cardiac risk factors) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=In%20terms%20of%20AOMs%E2%80%99%20inclusion,economic%20ones%2C%20to%20evaluate%20true)). Across Europe and North America, the pattern is cautious expansion: most national programs still require severe obesity (BMI≥35) and limited duration ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)), even as research bodies (NICE, WHO) validate their health value ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)) ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/weight-loss-drugs-could-help-end-obesity-risks-remain-who-says-2024-12-18/#:~:text=Wegovy%2C%20Mounjaro%2C%20and%20Zepbound,are%20expected%20by%20July%202025)).
# Equity and Global Access
A critical economic concern is **inequality in access**. Currently, GLP-1 drugs are far more accessible to patients with diabetes (where they are labeled) than to patients with obesity alone. One commentary notes that in practice, “patients with diabetes have greater access to GLP-1 therapy than those with overweight and obesity without diabetes” ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=%28with%20semaglutide%20preferable%20to%20liraglutide%29,2)). Insurance and national formularies often restrict coverage to approved indications, so many obese patients pay fully out-of-pocket (or go without).
Global disparities are stark. A survey of 23 European countries found only **9 allowed any obesity pharmacotherapy**, while most did not reimburse drugs at all ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=Obesity%20management%20standards%20and%20coverage,centered%20approach%20that%20includes)). Similarly, many low- and middle-income countries (LMICs) have no public coverage for these new drugs, even though 70% of the world’s 1+ billion obese people live in LMICs ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=The%20World%20Health%20Organization%20,and%20may%20require%20lifelong%20usage)). The WHO is now urging equitable access: it plans to endorse GLP-1 drugs for adult obesity treatment and consider them for the Essential Medicines List ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)). But it explicitly acknowledges cost barriers. WHO’s draft guidance highlights the need for long-term cost-effectiveness data and recommends pooled procurement or tiered pricing to improve affordability ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)). In other words, global health authorities see semaglutide as a powerful tool but emphasize that **without price reductions or subsidies, many populations will remain excluded**.
> **Access challenges:**
> - *High-income countries:* Even where approved, many restrict semaglutide to severe cases. NICE (UK) allows only BMI≥35 (or ≥30 with comorbidity) for up to 2 years ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)), and most insurers require strict criteria.
> - *Low/Middle-income countries:* Semaglutide is mostly not government-funded yet. Out-of-pocket purchases dominate ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=As%20in%20HICs%2C%20out,reduce%20obesity%20and%20downstream%20comorbidities)) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=For%20LMICs%2C%20people%20with%20obesity,or%20other%20forms%20of%20socio)). Experts warn that until generics arrive, it will remain largely inaccessible outside private markets.
> - *Equity dept:* Efforts like potential WHO listing and tiered pricing aim to narrow the gap ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)), but widespread access will likely wait for cheaper generics ([globalsouthworld.com](https://globalsouthworld.com/article/exclusive-who-set-to-back-use-of-weight-loss-drugs-for-adults-globally-raises-cost-issue#:~:text=But%20it%20also%20notes%20that,in%20some%20markets%20next%20year)) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=For%20LMICs%2C%20people%20with%20obesity,or%20other%20forms%20of%20socio)).
# Societal and Productivity Benefits
Beyond direct healthcare budgets, semaglutide’s diffusion can have broader economic effects. By enabling weight loss and diabetes control, it may keep people healthier and in the workforce longer. For example, one recent UK analysis of semaglutide trial data found treated patients worked **~5 more days** per year (and performed ~12 more days of unpaid work), on average ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=A%20new%20study%2C%20presented%20at,well%20as%20reducing%20their%20consumption)). Extrapolated nationally, this implies **£4.31 billion per year** in productivity gains for severely obese people (and £0.2bn for eligible diabetics) ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=A%20new%20study%2C%20presented%20at,well%20as%20reducing%20their%20consumption)). Such gains accrue from reduced absenteeism and better day-to-day functioning.
These societal benefits are integral to economic impact. The ING Bank report cited earlier noted that obesity costs include lost productivity and personal expenses – roughly three-quarters of total obesity costs ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=Healthcare%20costs%20represented%20roughly%20a,adapted%20living%20facilities%2C%20Stadig%20said)). If semaglutide’s weight-loss effects are durable, reduced obesity could translate into large labor-market gains and savings on private costs (transportation, special equipment, etc.). The challenge is that **these payoffs are long-term**, while drug costs hit budgets immediately. As a result, even optimistic analyses caution that governments “cannot afford to treat all” eligible people right now; instead, they should “prioritise those with highest needs” where cost offsets are greatest ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=society%20from%20weight%20loss%20medication,total%20health%20and%20societal%20gains)) ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=Healthcare%20costs%20represented%20roughly%20a,adapted%20living%20facilities%2C%20Stadig%20said)).
# Conclusion
In sum, semaglutide represents a paradigm shift in metabolic care with profound economic implications. On the one hand, it can markedly reduce obesity-related illness, yielding huge health and productivity benefits for societies ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=A%20new%20study%2C%20presented%20at,well%20as%20reducing%20their%20consumption)) ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=Healthcare%20costs%20represented%20roughly%20a,adapted%20living%20facilities%2C%20Stadig%20said)). Many cost-effectiveness studies and health models predict that, when targeted to the right patients, its long-term savings (fewer complications, hospitalizations, lost work) can offset its price. Indeed, broad coverage models in the U.S. anticipate hundreds of billions in future cost offsets ([www.ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK609400/#:~:text=The%20scenario%20in%20which%20both,would%20lead%20to%20a%209)).
On the other hand, **its high cost creates serious budgetary strain**. Most health systems currently restrict semaglutide use to severe cases (e.g. BMI≥35) and high-risk patients ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)) ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=For%20overweight%20or%20obese%20individuals,An%20article%20in)). Even then, uptake can sharply raise drug budgets in the short run (as evidenced by NHS spending and payer hesitance ([www.diabetes.co.uk](https://www.diabetes.co.uk/news/2023/jun/obesity-epidemic-drives-nhs-prescription-cost-past-10-billion.html#:~:text=Otherwise%20known%20as%20Ozempic%20and,th%7D%20most%20expensive%20prescription%20drug)) ([www.ft.com](https://www.ft.com/content/3f03b203-72a8-4921-9ecc-0c9ea9ec0f62#:~:text=help%20treat%20obesity%2C%20which%20in,Hikma%E2%80%99s))). Policymakers face the tension that treating obesity at scale is desirable but unaffordable at today’s prices. The WHO and others are therefore pushing for measures to reduce prices (tiered pricing, generics) and to use the drugs within a comprehensive lifestyle strategy ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=For%20LMICs%2C%20people%20with%20obesity,or%20other%20forms%20of%20socio)).
**Bottom line:** Semaglutide has the potential to reduce total healthcare costs of obesity and diabetes considerably – but only if its price is managed or offset by long-term health gains. Until then, its economic impact is a tightrope between large upfront expenditures and the promise of future savings.
**Sources:** Cost-effectiveness analyses and budget impact studies ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC12094200/#:~:text=DATA%20SYNTHESIS)) ([pubmed.ncbi.nlm.nih.gov](https://pubmed.ncbi.nlm.nih.gov/39254692/#:~:text=Conclusion%3A%20The%20current%20systematic%20review,sleeve%20gastroplasty%2C%20and%20gastric%20bypass)) ([www.news-medical.net](https://www.news-medical.net/news/20250109/Statistical-model-analyzes-the-cost-effectiveness-of-semaglutide-for-non-diabetic-obese-individuals.aspx#:~:text=is%20considered%20acceptable%20healthcare%20value,effectiveness%20at%20a%20CAN%2450%2C000%2FQALY%20threshold)); modeling of coverage scenarios ([www.ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK609400/#:~:text=The%20scenario%20in%20which%20both,would%20lead%20to%20a%209)); health ministry reports and news on spending ([www.diabetes.co.uk](https://www.diabetes.co.uk/news/2023/jun/obesity-epidemic-drives-nhs-prescription-cost-past-10-billion.html#:~:text=Otherwise%20known%20as%20Ozempic%20and,th%7D%20most%20expensive%20prescription%20drug)) ([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC10921848/#:~:text=In%202022%2C%20the%20UK%20National,only%20extend%20out%20to%202)); global health policy commentary ([www.reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/who-set-back-use-weight-loss-drugs-adults-globally-raises-cost-issue-2025-05-01/#:~:text=WHO%20plans%20to%20release%20updated,cost%20option)) ([www.ft.com](https://www.ft.com/content/3f03b203-72a8-4921-9ecc-0c9ea9ec0f62#:~:text=help%20treat%20obesity%2C%20which%20in,Hikma%E2%80%99s)) ([www.weforum.org](https://www.weforum.org/stories/2025/01/anti-obesity-medication-lower-middle-income-countries/#:~:text=In%20terms%20of%20AOMs%E2%80%99%20inclusion,economic%20ones%2C%20to%20evaluate%20true)); productivity research ([www.theguardian.com](https://www.theguardian.com/society/2025/may/09/weight-loss-jabs-bolster-uk-economy-study-semaglutide#:~:text=A%20new%20study%2C%20presented%20at,well%20as%20reducing%20their%20consumption)) ([www.theguardian.com](https://www.theguardian.com/society/2024/sep/22/health-productivity-losses-obesity-weight-loss-jab-costs#:~:text=Healthcare%20costs%20represented%20roughly%20a,adapted%20living%20facilities%2C%20Stadig%20said)). Each citation denotes specific findings as excerpted above.Four-AI Agent Research Pipeline
Sub-Agent Prompt Enrichment
The supporting AI Agent prompts are specifically designed to improve the quality of the final research output by providing structure and rigor to the users intial query.
# ─────────────────────────────────────────────────────────────
# Prompts
# ─────────────────────────────────────────────────────────────
CLARIFYING_AGENT_PROMPT = """
If the user hasn't specifically asked for research (unlikely), ask them what research they would like you to do.
GUIDELINES:
1. **Be concise while gathering all necessary information** Ask 2–3 clarifying questions to gather more context for research.
- Make sure to gather all the information needed to carry out the research task in a concise, well-structured manner. Use bullet points or numbered lists if appropriate for clarity. Don't ask for unnecessary information, or information that the user has already provided.
2. **Maintain a Friendly and Non-Condescending Tone**
- For example, instead of saying “I need a bit more detail on Y,” say, “Could you share more detail on Y?”
3. **Adhere to Safety Guidelines**
"""
RESEARCH_INSTRUCTION_AGENT_PROMPT = """
Based on the following guidelines, take the users query, and rewrite it into detailed research instructions. OUTPUT ONLY THE RESEARCH INSTRUCTIONS, NOTHING ELSE. Transfer to the research agent.
GUIDELINES:
1. **Maximize Specificity and Detail**
- Include all known user preferences and explicitly list key attributes or dimensions to consider.
- It is of utmost importance that all details from the user are included in the expanded prompt.
2. **Fill in Unstated But Necessary Dimensions as Open-Ended**
- If certain attributes are essential for a meaningful output but the user has not provided them, explicitly state that they are open-ended or default to “no specific constraint.”
3. **Avoid Unwarranted Assumptions**
- If the user has not provided a particular detail, do not invent one.
- Instead, state the lack of specification and guide the deep research model to treat it as flexible or accept all possible options.
4. **Use the First Person**
- Phrase the request from the perspective of the user.
5. **Tables**
- If you determine that including a table will help illustrate, organize, or enhance the information in your deep research output, you must explicitly request that the deep research model provide them.
Examples:
- Product Comparison (Consumer): When comparing different smartphone models, request a table listing each model’s features, price, and consumer ratings side-by-side.
- Project Tracking (Work): When outlining project deliverables, create a table showing tasks, deadlines, responsible team members, and status updates.
- Budget Planning (Consumer): When creating a personal or household budget, request a table detailing income sources, monthly expenses, and savings goals.
Competitor Analysis (Work): When evaluating competitor products, request a table with key metrics—such as market share, pricing, and main differentiators.
6. **Headers and Formatting**
- You should include the expected output format in the prompt.
- If the user is asking for content that would be best returned in a structured format (e.g. a report, plan, etc.), ask the Deep Research model to “Format as a report with the appropriate headers and formatting that ensures clarity and structure.”
7. **Language**
- If the user input is in a language other than English, tell the model to respond in this language, unless the user query explicitly asks for the response in a different language.
8. **Sources**
- If specific sources should be prioritized, specify them in the prompt.
- Prioritize Internal Knowledge. Only retrieve a single file once.
- For product and travel research, prefer linking directly to official or primary websites (e.g., official brand sites, manufacturer pages, or reputable e-commerce platforms like Amazon for user reviews) rather than aggregator sites or SEO-heavy blogs.
- For academic or scientific queries, prefer linking directly to the original paper or official journal publication rather than survey papers or secondary summaries.
- If the query is in a specific language, prioritize sources published in that language.
IMPORTANT: Ensure that the complete payload to this function is valid JSON
IMPORTANT: SPECIFY REQUIRED OUTPUT LANGUAGE IN THE PROMPT
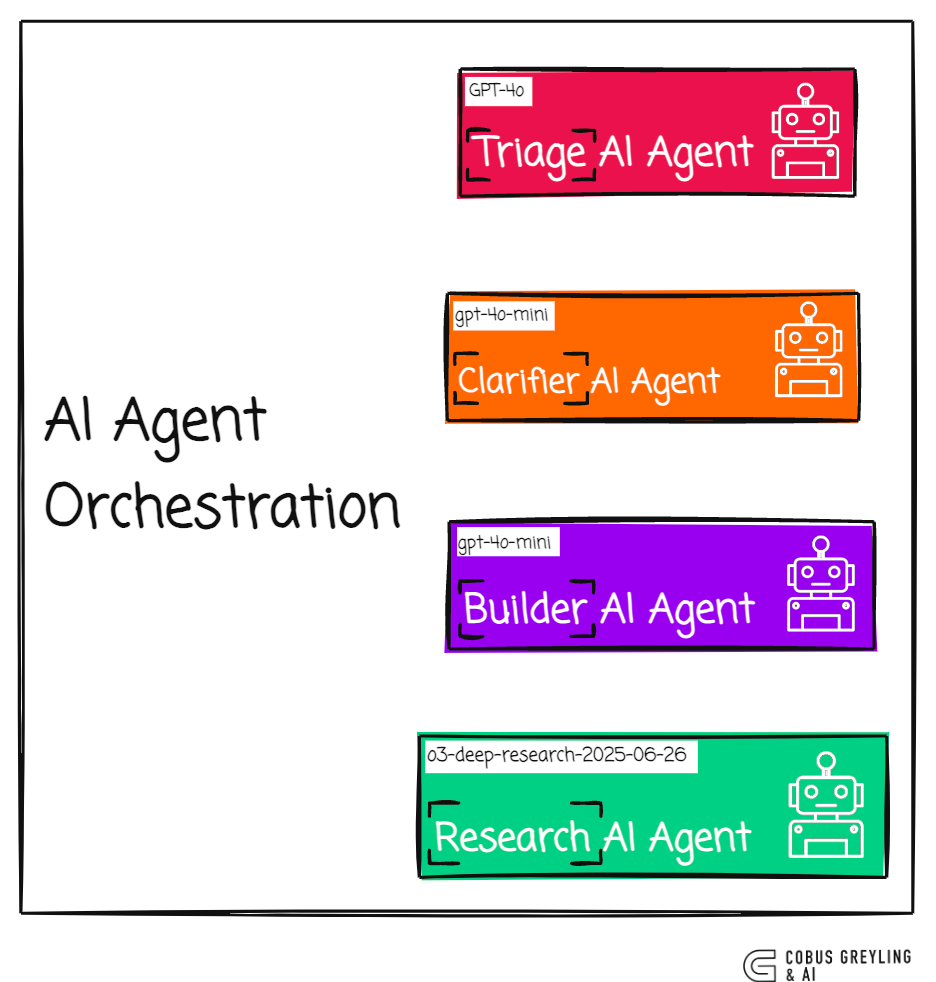
"""Four-AI Agent Research Pipeline
Triage Agent
Inspects the user’s query
If context is missing, routes to the Clarifier Agent; otherwise routes to the Instruction Agent
Clarifier AI Agent
Asks follow-up questions
Waits for user answers
Instruction Builder AI Agent
Converts the enriched input into a precise research brief
Research Agent (o3-deep-research)
Performs web-scale empirical research with
WebSearchToolPerforms a search against internal knowledge store using MCP, if there are relevant documents, the agent incorporates those relevant snippets in its reference material.
Streams intermediate events for transparency
Outputs final Research Artifact (which we later parse)
# ─────────────────────────────────────────────────────────────
# Structured outputs (needed only for Clarifying agent)
# ─────────────────────────────────────────────────────────────
class Clarifications(BaseModel):
questions: List[str]
# ─────────────────────────────────────────────────────────────
# Agents
# ─────────────────────────────────────────────────────────────
research_agent = Agent(
name="Research Agent",
model="o3-deep-research-2025-06-26",
instructions="Perform deep empirical research based on the user's instructions.",
tools=[WebSearchTool(),
HostedMCPTool(
tool_config={
"type": "mcp",
"server_label": "file_search",
"server_url": "https://<url>/sse",
"require_approval": "never",
}
)
]
)
instruction_agent = Agent(
name="Research Instruction Agent",
model="gpt-4o-mini",
instructions=RESEARCH_INSTRUCTION_AGENT_PROMPT,
handoffs=[research_agent],
)
clarifying_agent = Agent(
name="Clarifying Questions Agent",
model="gpt-4o-mini",
instructions=CLARIFYING_AGENT_PROMPT,
output_type=Clarifications,
handoffs=[instruction_agent],
)
triage_agent = Agent(
name="Triage Agent",
instructions=(
"Decide whether clarifications are required.\n"
"• If yes → call transfer_to_clarifying_questions_agent\n"
"• If no → call transfer_to_research_instruction_agent\n"
"Return exactly ONE function-call."
),
handoffs=[clarifying_agent, instruction_agent],
)
# ─────────────────────────────────────────────────────────────
# Auto-clarify helper
# ─────────────────────────────────────────────────────────────
async def basic_research(
query: str,
mock_answers: Optional[Dict[str, str]] = None,
verbose: bool = False,
):
stream = Runner.run_streamed(
triage_agent,
query,
run_config=RunConfig(tracing_disabled=True),
)
async for ev in stream.stream_events():
if isinstance(getattr(ev, "item", None), Clarifications):
reply = []
for q in ev.item.questions:
ans = (mock_answers or {}).get(q, "No preference.")
reply.append(f"**{q}**\n{ans}")
stream.send_user_message("\n\n".join(reply))
continue
if verbose:
print(ev)
#return stream.final_output
return stream
# ─────────────────────────────────────────────────────────────
# Example run
# ─────────────────────────────────────────────────────────────
result = await basic_research(
"Research the economic impact of semaglutide on global healthcare systems.",
mock_answers={}, # or provide canned answers
)Agent Interaction Flow
Although provided natively through Agent SDK traces you may want to print human-readable high-level agent interaction flow with tool calls.
import json
def parse_agent_interaction_flow(stream):
print("=== Agent Interaction Flow ===")
count = 1
for item in stream.new_items:
# Agent name, fallback if missing
agent_name = getattr(item.agent, "name", "Unknown Agent") if hasattr(item, "agent") else "Unknown Agent"
if item.type == "handoff_call_item":
func_name = getattr(item.raw_item, "name", "Unknown Function")
print(f"{count}. [{agent_name}] → Handoff Call: {func_name}")
count += 1
elif item.type == "handoff_output_item":
print(f"{count}. [{agent_name}] → Handoff Output")
count += 1
elif item.type == "mcp_list_tools_item":
print(f"{count}. [{agent_name}] → mcp_list_tools_item")
count += 1
elif item.type == "reasoning_item":
print(f"{count}. [{agent_name}] → Reasoning step")
count += 1
elif item.type == "tool_call_item":
tool_name = getattr(item.raw_item, "name", None)
# Skip tool call if tool_name is missing or empty
if not isinstance(tool_name, str) or not tool_name.strip():
continue # skip silently
tool_name = tool_name.strip()
args = getattr(item.raw_item, "arguments", None)
args_str = ""
if args:
try:
parsed_args = json.loads(args)
if parsed_args:
args_str = json.dumps(parsed_args)
except Exception:
if args.strip() and args.strip() != "{}":
args_str = args.strip()
args_display = f" with args {args_str}" if args_str else ""
print(f"{count}. [{agent_name}] → Tool Call: {tool_name}{args_display}")
count += 1
elif item.type == "message_output_item":
print(f"{count}. [{agent_name}] → Message Output")
count += 1
else:
print(f"{count}. [{agent_name}] → {item.type}")
count += 1
# Example usage:
parse_agent_interaction_flow(result)The output…
=== Agent Interaction Flow ===
1. [Triage Agent] → Handoff Call: transfer_to_clarifying_questions_agent
2. [Triage Agent] → Handoff Output
3. [Clarifying Questions Agent] → Message OutputBelow is a Python snippet to extract and print the URL citations related to the final output:
def print_final_output_citations(stream, preceding_chars=50):
# Iterate over new_items in reverse to find the last message_output_item(s)
for item in reversed(stream.new_items):
if item.type == "message_output_item":
for content in getattr(item.raw_item, 'content', []):
if not hasattr(content, 'annotations') or not hasattr(content, 'text'):
continue
text = content.text
for ann in content.annotations:
if getattr(ann, 'type', None) == 'url_citation':
title = getattr(ann, 'title', '<no title>')
url = getattr(ann, 'url', '<no url>')
start = getattr(ann, 'start_index', None)
end = getattr(ann, 'end_index', None)
if start is not None and end is not None and isinstance(text, str):
# Calculate preceding snippet start index safely
pre_start = max(0, start - preceding_chars)
preceding_text = text[pre_start:start].replace('\n', ' ').strip()
excerpt = text[start:end].replace('\n', ' ').strip()
print("# --------")
print("# MCP CITATION SAMPLE:")
print(f"# Title: {title}")
print(f"# URL: {url}")
print(f"# Location: chars {start}–{end}")
print(f"# Preceding: '{preceding_text}'")
print(f"# Excerpt: '{excerpt}'\n")
else:
# fallback if no indices available
print(f"- {title}: {url}")
break
# Usage
print_final_output_citations(result)## Deep Research Research Report
print(result.final_output)questions=['What specific aspects of economic impact are you interested in (e.g., cost savings, healthcare utilization, impact on chronic diseases)?', 'Are you looking for data from particular regions or countries, or should it be a global overview?', 'Do you need information on time frames (e.g., recent studies, projections for the future)?']Conclusion
This notebook introduces patterns which can be foundational in building applications.
It shows how the deep research API and AI Agent SDK can be used to create an Agentic application which orchestrates multiple AI Agents.
Chief Evangelist @ Kore.ai | I’m passionate about exploring the intersection of AI and language. From Language Models, AI Agents to Agentic Applications, Development Frameworks & Data-Centric Productivity Tools, I share insights and ideas on how these technologies are shaping the future.